AstraZeneca's 'semitinib' approved in China
On May 8, the State Drug Administration website showed that AstraZeneca's MEK1/2 inhibitor, semitinib, was approved for the treatment of patients aged 3 years and older with symptomatic and/or progressive, inoperable neurofibromatosis type I (NF1)-associated plexiform neurofibromas (PN).
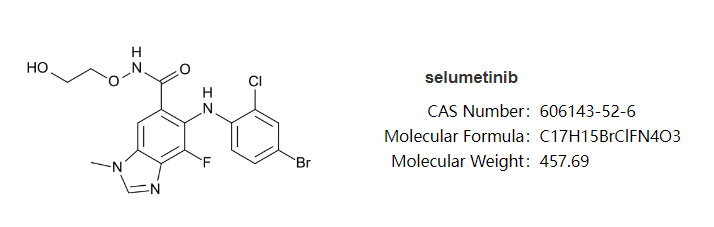
The mitogen-activated protein kinase kinase 1 and 2 (MEK1/2) inhibitor semetinib, also known as selumetinib, was created by Array BioPharma, a Pfizer subsidiary, and received its initial commercial approval in April 2020 under the brand name Koselugo.In December 2003, AstraZeneca and Array BioPharma agreed into a partnership for the development and global commercialization of semitinib. In July 2017, AstraZeneca engaged into a similar partnership with Merck Sharp & Dohme.
Results from a Phase II clinical trial served as the foundation for semitinib's prior FDA approval. The goal of the 50-patient trial was to assess semitinib's effectiveness and safety in the treatment of children patients with inoperable NF1. The objective remission rate (ORR) was the trial's main outcome.
According to the findings, individuals receiving twice-daily smectinib had an ORR of 66% and had all had partial remission. Additionally, 82% of patients experienced remissions lasting 12 months or longer.
To further assess the safety and efficacy of semitinib, AstraZeneca started a phase III clinical trial (KOMET) of the drug in China in December 2021. 146 participants are enrolled in the trial, which is anticipated to be finished by November 2023.
Anywhere in the body, PN tumors affect the nerve sheath, the covering that surrounds nerve fibers.A uncommon and progressive condition called NF1 is brought on by changes or flaws in particular genes.An estimated 1 in 3,000 people are predicted to have NF1, which is often identified in early childhood.
A total of four MEK inhibitors, including semitinib (AstraZeneca/Mercedon), binitinib (Pfizer/Pierre Fabre/Ono Pharmaceutical), cobimetinib (Roche/Exelixis), and trametinib (Japan Tobacco/Novartis), have been authorized for marketing globally as of this point. Trametinib began selling in China in December 2019. Several domestic pharmaceutical firms have developed MEK inhibitors, including KZP, Hengrui Pharmaceuticals, Fultronics, and Zhengda Tianqing. Trametinib, developed by KZP, was announced to be listed in China on January 29 of this year, and SHR7390 and FCN-159, developed by Hengrui Pharmaceuticals and Fultronics, respectively, are in the Phase II clinical stage.
Copyright © 2023 PHARMCUBE. All Rights Reserved.

