CAS No.: 1143-38-0 Irritant contact dermatitis produced by the topical contact irritant anthralin induces hair regrowth, with remission rates of 75% for limited pemphigus and 25% for alopecia totalis in uncontrolled studies. Dithranol topical topical application has immunosuppressive effects and is now used in the treatment of pemphigus vulgaris, including in children, and can be used in pediatric patients with severe pemphigus vulgaris. The drug has immunosuppressive and anti-inflammatory effects when applied topically. Standard therapy is short-term contact therapy with 0.5% to 1% anthraquinone, with applications ranging from a few minutes to overnight, with an onset of action of 3 months, and discontinuation if ineffective. The most common adverse reactions are folliculitis, contact dermatitis, and hyperpigmentation.
Methotrexate
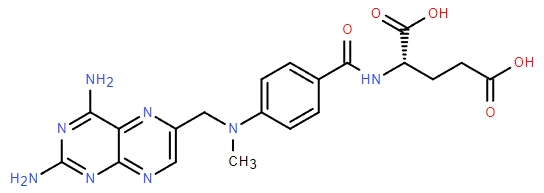
Chemical Name: Methotrexate
Molecular Formula: C20H22N8O5
Formula Weight: 454.45
CAS No.: 59-05-2
Effective in a range of inflammatory reactions and autoimmune disorders, it is now used as monotherapy for the treatment of pemphigus vulgaris and as an adjunct to corticosteroids.Methotrexate can be used before or after hormones, or alone after hormone therapy has failed. It is indicated for the treatment of severe pemphigus vulgaris in adults and less frequently in children.Joly and Droitcourt et al. reported satisfactory hair regrowth after methotrexate application in approximately 64% and 70% of adult cases, respectively.A systematic review and Meta-analysis of previous studies using methotrexate for the treatment of pemphigus vulgaris by Phan et al. showed that methotrexate was significantly effective in patients with severe pemphigus vulgaris. Adults appear to be more responsive to methotrexate treatment than children; the combination of methotrexate and corticosteroids is more effective than methotrexate alone; and Public No. Synthetic Drugs found that a large percentage of relapses were associated with tapering of methotrexate treatment, and therefore treating physicians should consider extending the duration of methotrexate therapy if the treatment is well tolerated, but the optimal duration of methotrexate therapy has not been However, the optimal duration of methotrexate therapy has not been determined. The most common adverse effects of methotrexate identified to date are hepatic impairment, gastrointestinal reactions, and infections; the relative risk of these adverse effects is low, but highlights the importance of regular monitoring. To date, most of the available evidence consists of retrospective observational studies, and there is a lack of prospective controlled trials evaluating the efficacy and risks of methotrexate for the treatment of pemphigus vulgaris.
Cyclosporine

Chemical Name: Cyclosporine
Molecular Formula: C62H111N11O12
Formula Weight: 1202.61
CAS No.: 59865-13-3
A calcineurin phosphatase inhibitor which reduces the activity of the immune system by specifically inhibiting T-cell activity; during cyclosporine treatment of pemphigus vulgaris, there is a decrease in immune system cells (including T-cells, CD4+ T-cells, CD8+ T-cells, and Langerhans cells) in the hair follicles. On the other hand, one of the known adverse effects of cyclosporine is hirsutism, suggesting that this drug has a role in promoting hair regrowth, which contributes to its therapeutic effect on pemphigus vulgaris]. Cyclosporine can be used as a second-line drug in hormone-responsive but hormone-dependent patients to halt disease progression and induce hair regrowth. Current literature estimates the response rate to cyclosporine to be between 33% and 55%; however, this has not been evaluated in randomized controlled trials.Lai et al. demonstrated that 4 mg/(kg-d) cyclosporine monotherapy for 3 months was moderately effective in inducing remission of pemphigus vulgaris, and that the combination of cyclosporine with glucocorticosteroids may further improve efficacy. The use of cyclosporine at too high a dose or for too long a period of time carries the risk of adverse effects, particularly nephrotoxicity, neurotoxicity, hypertension, and hyperlipidemia, and it is difficult to control the balance between its efficacy and dosing.
Chemical Name: Baricitinib
Molecular Formula: C16H17N7O2S
Formula Weight: 371.42
CAS No.: 1187594-09-7

Eli Lilly Development. Clinically used for the treatment of a variety of inflammatory and autoimmune diseases, including rheumatoid arthritis (RA), psoriasis, diabetic nephropathy, atopic dermatitis (AD), systemic lupus erythematosus (SLE), etc.20 In June 2022, the FDA approved the first JAK inhibitor, baricitinib (Olumiant), for the treatment of adult pemphigus vulgaris, and in March 2023, baricitinib was NMPA approved and domestically marketed for the treatment of severe baldness in adults.
Drugs in development
Jaktinib
is being developed in-house by Zejing Pharmaceuticals. Tablets of the drug are currently undergoing Phase II clinical trials in six indications, including high-risk myelofibrosis, active ankylosing spondylitis, severe pemphigus vulgaris, idiopathic pulmonary fibrosis, moderate-to-severe plaque psoriasis and moderate-to-severe atopic dermatitis.
ATI-50002(ifidancitinib)
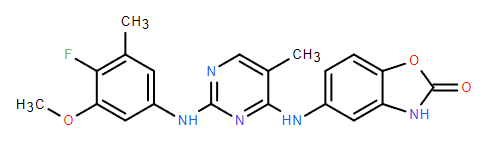
Chemical Name: ATI-50002
Molecular Formula: C20H18FN5O3
Formula Weight: 395.39
CAS No.: 1236667-40-5
Ifidancitinib
Developed by Aclaris Therapeutics, Ifidancitinib (ATI-50002) is another dual JAK1 and JAK3 inhibitor for the treatment of pemphigus vulgaris in oral and topical formulations and is currently in Phase 2 clinical phase.
LH-8
An experimental topical agent developed by Legacy Healthcare in Switzerland. Among the active substances present in Coacillium are a variety of flavonoids, polyphenols, and methylxanthines related to achieve multiple mechanisms of action (i.e., multi-targeting) to simultaneously reverse the immune-inflammatory response and its deleterious effects on the hair follicle and adjacent dermal tissue. Phase II/III, double-blind, carrier-controlled, randomized, multicenter trials are currently underway to evaluate the efficacy and safety of Coacillium Dermal Liquid for the treatment of children and adolescents with moderate (25% ~ 50% baldness) to severe (>50% baldness) baldness.deuruxolitinib (CTP-543)
Chemical Name: CTP-543
Molecular Formula: C17H18N6
Formula Weight: 306.37
CAS No.: 1513883-39-0
Deuruxolitinib.
Concert Pharmaceuticals developed and was acquired by Indian drugmaker Sun Pharma in January 2023.On July 8, 2020, the FDA granted Breakthrough Therapy Designation and Fast Track Designation to CTP-543 for the treatment of adults with moderate to severe pemphigus vulgaris for the treatment of pemphigus vulgaris. The company is evaluating the efficacy and safety of deuruxolitinib in adult patients with moderate to severe pemphigus vulgaris in its THRIVE- Phase III clinical program.Concert reported positive backline data from the clinical trial.On October 6, 2023, India's Sun Pharmaceuticals announced that Deuruxolitinib's New Drug Application for the treatment of moderate to severe pemphigus vulgaris was accepted by the FDA.
EQ101.
Equillium developed a tri-specific inhibitor of IL-2, IL-9 and IL-15, three inflammatory cytokines implicated in a wide range of diseases.EQ101 has also been shown to be well tolerated and has a favorable safety profile, with no drug-related adverse effects or dose-limiting toxicities. The drug is currently used intravenously and a subcutaneous formulation is under development. The drug is currently in a Phase II study for the treatment of pemphigus vulgaris, a dermatologic autoimmune disease.
MAX 40070
A topical JAK/Tyk2 inhibitor developed by MaxinovelPharmaceuticals. Preclinical studies have demonstrated that topical administration of MAX-40070 achieves effective exposure in skin tissue while maintaining low systemic exposure.MAX-40070 has the potential to minimize the systemic side effects of oral JAK inhibitors and ultimately demonstrate a high benefit/risk ratio through clinical trials, which may ultimately provide strong evidence in support of its wider use in the treatment of dermatological autoimmune diseases provide strong evidence. The drug is currently in Phase I development for the treatment of pemphigus vulgaris.
ENERGI-F701
Developed by Energenesis Biomedical, Taiwan. Designed to treat hair loss in men and women. By enhancing AMPK activity, ENERGI - f701 can increase ATP in human hair follicle dermal papilla cells (HFDPCs), thereby slowing down the aging rate and prolonging the hair cycle (). Meanwhile, animal experiments simulating baldness with dihydrotestosterone (DHT) showed that energy - f701 was able to attenuate the effect of DHT on inhibiting hair growth.Farudodstat
Chemical Name: Farudodstat
Molecular Formula: C19H14F2N2O3
Formula Weight: 356.32
CAS No.: 1035688-66-4 Developed by ASLAN Pharmaceuticals.On May 18, 2023, ASLAN Pharmaceuticals Pte Ltd announced the use of Farudodstat, an oral DHODH inhibitor, in a Phase 2a proof-of-concept study in adult patients suffering from Alopecia Areata (AA).The study, titled "FAST-AA " (FArudodstat STudy in Alopecia Areata) will investigate the efficacy and safety of farudodstat in at least 50% of patients with alopecia areata of the scalp.FAST-AA will enroll patients in at least 20 sites across the U.S. Top-line data are expected to be available from the first 12-week treatment period in Q1 2024.Bimatoprost
Chemical Name: Bimatoprost
Molecular Formula: C25H37NO4
Formula Weight: 415.57
CAS No.: 155206-00-1
Bimatoprost
Bimatoprost is a globally recognized drug that is effective in promoting eyelash growth and was approved by the FDA in 2008 for the treatment of glaucoma. Currently, Ahmed Hassan Nouh MD, Allergan and others are developing it for the treatment of hair loss in Phase 1-2 clinical stage.
KL130008
KL130008 capsule is a selective inhibitor targeting JAK1 and JAK2 kinases, which has been approved for rheumatoid arthritis indication and is in phase II clinical trial.According to the official website of CDE, KL130008 capsule of Kelun Pharmaceuticals' subsidiary, Kelun Botai, Sichuan, has been granted with implied consent to be used for the treatment of severe pemphigus vulgaris.
ADX-914
Developed by Q32 Bio.2022 On August 15, 2022, Q32 Bio, a clinical-stage biotechnology company, and Horizon Therapeutics plc announced that they have entered into a collaboration and option agreement for the development of ADX-914 for the treatment of autoimmune disorders.ADX-914 is a fully human anti-IL-7Rα antibody, which is used in the treatment of autoimmune disorders by blocking signaling mediated by IL-7 and TSLP-mediated signaling to re-regulate adaptive immune function.Upadacitinib
Chemical Name: Upadacitinib
Molecular Formula: C17H19F3N6O
Formula Weight: 380.37
CAS No.: 1310726-60-3
Upadacitinib
AbbVie Development. Upadacitinib has been approved by the U.S. FDA for seven indications in the areas of gastroenterology, dermatology, and rheumatology, according to the second quarter 2023 earnings report. The drug has also been approved for multiple indications in China, including moderate-to-severe atopic dermatitis, rheumatoid arthritis, active psoriatic arthritis, ulcerative colitis, and Crohn's disease.In 2023, the official website of China's State Pharmaceutical Administration's Drug Evaluation Center announced that AbbVie's filing of Upadacitinib tablets was granted implied approval for a new clinical trial to be developed for the treatment of severe pemphigus.Etrasimod
Chemical Name: Etrasimod
Molecular Formula: C26H26F3NO3
Formula Weight: 457.48
CAS No.: 1206123-37-6
Etrasimod
Etrasimod, developed by Arena Pharmaceuticals, which Pfizer completed its acquisition of in December 2021, is primarily used for the treatment of autoimmune diseases, including ulcerative colitis, Crohn's disease, and atopic dermatitis. Etrasimod is currently in Phase 3 for the treatment of moderately to severely active ulcerative colitis (UC), and is in Phase 2 clinical development for the treatment of pemphigus vulgaris.Ruxolitinib
Chemical Name: Ruxolitinib
Molecular Formula: C17H18N6
Formula Weight: 306.37
CAS No.: 941678-49-5
Ruxolitinib
Developed by Novartis, ruxolitinib was formally approved by the FDA in 2011 as the first drug for the treatment of myelofibrosis. ruxolitinib is being investigated for the treatment of pemphigus vulgaris by Incyte Corporation and others, and is still in clinical phase 2.Crisaborole
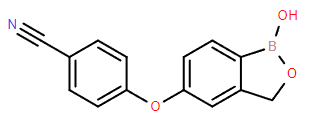
Chemical Name: Crisaborole
Molecular Formula: C14H10BNO3
Formula Weight: 251.05
CAS No.: 906673-24-3
Crisaborole.
was developed by Anacor and approved for marketing by the FDA on December 14, 2016 for the treatment of mild to moderate atopic dermatitis in children and adults.In June 2016, Pfizer acquired Anacor.Pfizer and Tufts Medical Center partnered to study the treatment of pemphigus vulgaris. The Phase 2 clinical study has been withdrawn due to the inability to identify subjects who met the inclusion criteria.
SHR0302.
A highly selective JAK1 inhibitor developed by Ruishi Biopharmaceuticals Ltd. The available data on similar JAK inhibitors globally suggest that they have some efficacy in the treatment of pemphigus vulgaris. A randomized, double-blind, placebo-controlled Phase III study evaluating the efficacy and safety of SHR0302 tablets in adults with pemphigus vulgaris is currently underway.Tofacitinib

Chemical Name: Tofacitinib
Molecular Formula: C16H20N6O
Formula Weight: 312.38
CAS No.: 477600-75-2
Tofacitinib
Developed by Pfizer. It is used primarily for the treatment of rheumatoid and chronic arthritis.Locks of Love, Yale University, and others are in Phase 2 clinical studies for the treatment of pemphigus vulgaris.Delgocitinib

Chemical Name: Delgocitinib
Molecular Formula: C16H18N6O
Formula Weight: 310.35
CAS No.: 1263774-59-9
Delgocitinib
On January 23, 2021, the PMDA approved Japan Tobacco's delgocitinib ointment for the treatment of atopic dermatitis in a Phase 2 clinical study initiated by LEO Pharma for the treatment of pemphigus vulgaris.Clascoterone

Chemical Name: Clascoterone
Molecular Formula: C24H34O5
Formula Weight: 402.52
CAS No.: 19608-29-8
Clascoterone
Developed by Cassiopea and approved by the U.S. FDA in 2020 for the treatment of acne.Cassiopea has announced positive Phase 2 clinical trial results for Clascoterone for the treatment of patients with androgenetic alopecia in April 2019, in addition to its Phase 3 clinical trial for the treatment of male patients with androgenetic alopecia.
KX-826
Developed by Pioneer Pharmaceuticals. KX-826 has initiated a long-term safety trial in China for the treatment of androgenetic alopecia, with first patient enrollment completed on July 19, 2023. The long-term safety trial was approved by the State Drug Administration of China on April 18, 2023 to proceed.
TDM-105795
Developed by Teclo Bio. Successfully completed the clinical phase I study at the end of December 2022 and expects to start the phase II clinical trial in mid-March 2023.















