Name:
Olutasidenib
CAS: 1887014-12-1
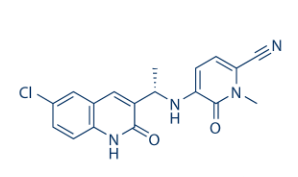
Develop Company: Rigel Pharmaceuticals
Indication: Acute myeloid leukemia
English trade name: REZLIDHIA
FDA approval date: December 1, 2022
Formulation: Capsules 150mg
2102-HEM-101 (NCT02719574) Test
Trial Information
147 adult patients with relapsed or resistant AML who had the IDH1 mutation were enrolled in the clinical trial.
Olutasidenib was given orally at a dose of 150 mg twice daily until the disease progressed, unacceptable toxicity developed, or a hematopoietic stem cell transplant was performed. The average length of treatment was 4.7 months (range: 0.1-26 months). Following olutasidenib therapy, sixteen patients underwent hematopoietic stem cell transplantation.
Trial results
In adult patients with recurrent or refractory AML having the IDH1 mutation, the combined rate of complete remission (CR) + complete remission with partial hematological (CRh) remission was 35%, with CR at 32% and CRh at 2.7%. The median length of remission was 25.9 months (95% CI: 13.5 months, not attained), with a median time to CR+CRh of 1.9 months (range: 0.9-5.6 months).
Rezlidhia showed good tolerance, with side effects characterized by symptoms and characteristics similar to those seen in treated AML patients. In 16% of individuals, fractionation syndrome was found, which could be controlled in most cases by discontinuing dosage and using corticosteroids. Hepatotoxicity, shown by increased liver function markers in 23% of patients, was manageable in most cases by dosage modification. By 56 days after baseline, 29 (34%) of the 86 patients who were dependent on red blood cell (RBC) and/or platelet transfusions were non-reliant on RBC and platelet transfusions. 39 (64%) of the 61 patients who did not require RBC and platelet transfusions at baseline were transfusion-independent for 56 days beyond baseline.
Olutasidenib synthetic route
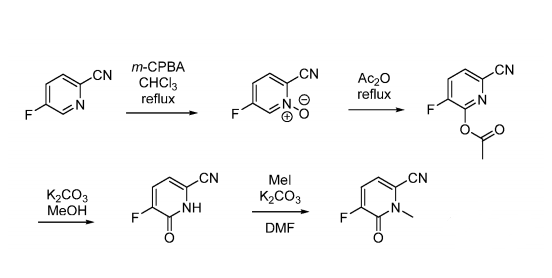
Reference: WO2016044789
FDA has approved leukemia treatment drugs
1. imatinib mesylateCAS: 220127-57-1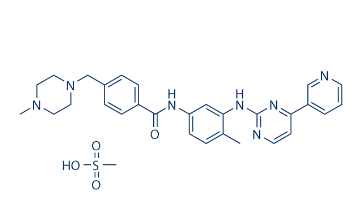
Indications: chronic myeloid leukemia, chronic eosinophilic leukemia, Acute lymphoblastic leukemia, myelodysplastic syndrome, bone marrow and aggressive systemic mastocytosis, extramedullary proliferation, gastrointestinal mesenchymal tumor, eosinophilia, augmented dermatofibrosarcoma.
Original research company: Novartis
FDA approval date: 2001/05/10
2.
DasatinibCAS: 302962-49-8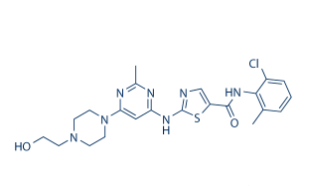
Indications: Acute lymphoblastic leukemia, chronic myeloid leukemia.
Originating company: Bristol-Myers Squibb
FDA approval date: 2006/06/28
3.
NilotinibCAS: 641571-10-0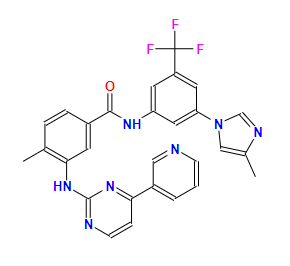
Indication: Treatment for patients with m chronic myeloid leukemia who are resistant or tolerant to the drug imatinib.
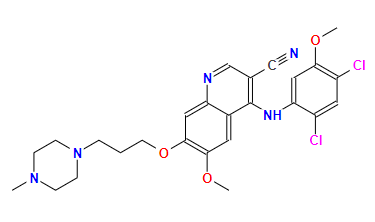
Indications: For the treatment of chronic phase, accelerated phase, or maternal cell phase chronic granulomatous leukemia (CML) that has become resistant or intolerant to prior tyrosine kinase inhibitor therapy, as well as Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) that has become resistant or intolerant to prior tyrosine kinase inhibitor therapy.
Originating company: Wyeth Pharmaceuticals
FDA approval date: 2012/09/04
5.
ibrutinibCAS: 936563-96-1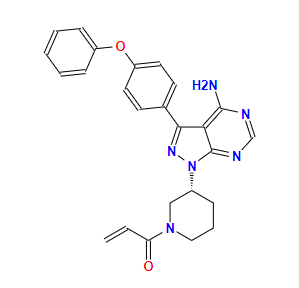
Indications: Chronic lymphocytic leukemia, cluster cell lymphoma, small lymphocytic lymphoma, graft-versus-host disease, Waldenstrom's macroglobulinemia, marginal zone lymphoma.
Originating company: Pharmacyclics
6.
idelalisibCAS: 1146702-54-6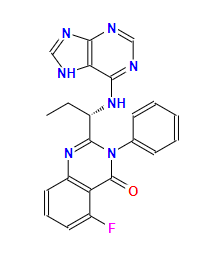
Indications: Treatment for relapsed chronic lymphocytic leukemia (CLL), relapsed follicular B non-Hodgkin's lymphoma (FL), and relapsed small lymphocytic lymphoma (SLL).
Original investigational company: Gilead
FDA approval date: 2014/07/23
7.
VenetoclaxCAS: 1257044-40-8
Indication: Treatment for patients with chronic lymphocytic leukemia carrying a deletion mutation in the 17p gene and who have received at least one prior therapy.
Original investigator: AbbVie
FDA approval date: 2016/04/11
8.
midostaurinCAS: 120685-11-2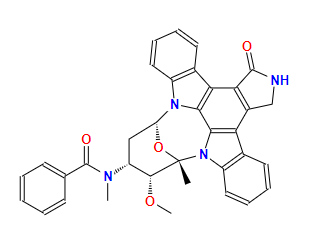
Indications: For the treatment of chronic phase, accelerated phase, or maternal cell phase chronic granulomatous leukemia (CML) that has become resistant or intolerant to prior tyrosine kinase inhibitor therapy, as well as Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) that has become resistant or intolerant to prior tyrosine kinase inhibitor therapy.
Original investigator company: Novartis
FDA approval date: 2017/04/28
9.
EnasidenibCAS: 1446502-11-9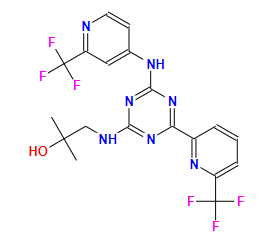
Treatment of relapsed or refractory acute myeloid leukemia in people with isocitrate dehydrogenase 2 (IDH2) gene mutations.
Originating company: Celgene
Indication: Adult patients with relapsed or refractory acute myeloid leukemia (R/R AML) who have a susceptible isocitrate dehydrogenase-1 (IDH1) mutation validated by a test (Abbott RealTime IDH1 companion diagnostic kit).
Original investigator: Agios Pharmaceuticals
11.
gilteritinibCAS: 1254053-43-4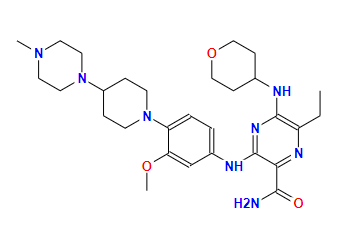
Indication: Treatment for adult patients with relapsed or refractory AML carrying FLT3 mutations.
Originating company: Astellas Pharma
12.
vincristineCAS: 57-22-7
Indications: Acute leukemia, especially in children












