The epilepsy treatment drug: Cenobamate
CAS No.:
913088-80-9

Original research company: SK Life Science
English trade names: XCOPRI (US), ONTOZRY (Europe)
Indications: Epilepsy
Date of market
Cenobamate was approved by the FDA on 2019-11-21 for the adjunctive treatment of partial-onset epilepsy in adults; on March 26, 2021, the EMA approved the marketing of cenobamate for the treatment of focal seizures, including those that eventually spread throughout the brain (secondary generalized); or in combination with other antiepileptic medications in combination for the treatment of adults with epilepsy who have received at least one prior antiepileptic medication.
FDA and EMA approval is based on two studies, Study013 and Study 017:
The 200 mg/day dose of cenobamate reduced median seizure frequency by 56% compared to 22% in the placebo group, according to data from Study #13. One post hoc analysis of the maintenance period revealed that 28% of patients in the cenobamate-treated group reported having no seizures, compared to 9% in the placebo group.
The three doses of cenobamate reduced median seizure frequency by 36%, 55%, and 55%, respectively, and by 24% in the placebo group, with statistically significant data difference; during the maintenance period, 4%, 11%, and 21% of patients in the 3-dose group reported zero seizures, compliant. Study 017: 1 6-week titration period and 1 12-week maintenance period; the study included 100 mg/day, 200 mg/day, and 400 mg/day doses
Japan and China have not yet ratified this.
The method of action
Although the precise method by which cenobamate exerts its therapeutic benefits is unknown, SK Biopharma thinks the medicine inhibits voltage-gated sodium currents to lessen repeated neuronal activity.
Compound Patents
WO2006112685, SK Life Science, applicant, application date April 21, 2006.
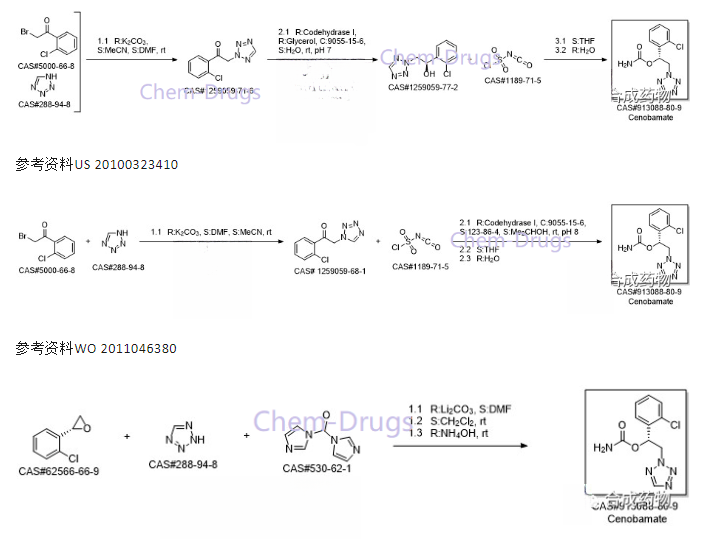
Synthesis route