Chemical Name:
enasidenib 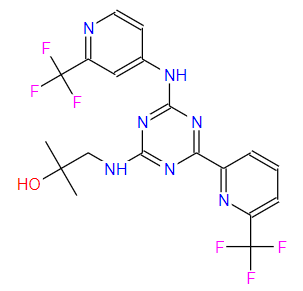
CAS:1446502-11-9
CAS: 1650550-25-6 (mesylate)(API)
English trade name: Idhifa
Indications: Leukemia
According to the data, as of January 2023, the number of leukemia patients in the world reached 300,000, and the number of leukemia patients in China is about 3/100,000 to 4/100,000, of which children account for 1/4 of the total number of patients.
Original Research Companies: Agios Pharmaceuticals; Celgene Corporation
On December 21, 2020, Servier announced that it had entered into an agreement to acquire the oncology business of Agios Pharmaceuticals, including its commercial, clinical and investigational stage oncology drugs.
On January 3, 2019, Bristol-Myers Squibb (BMS) announced the acquisition of Celgene Corporation for $74 billion.
Approved for marketing
FDA:August 1, 2017
For the treatment of relapsed or refractory acute myeloid leukemia in adults harboring mutations in the isocitrate dehydrogenase 2 (IDH2) gene.
EMA: Not approved at this time.
On April 28, 2016, granted orphan drug designation by the European Commission.
Acceptance of modifications to the agreed pediatric investigational plan for enasidenib under Regulation (EC) of the European Parliament and of the Council on October 28, 2022
PMDA:Not approved at this time.
NMPA:Not approved at this time.
Idhifa global sales
2.2 million dollars in 2019 (Agios), 6 million in 2020 (3 million dollars Agios, 3 million dollars BMS), 12 million dollars in 2021 (BMS)
