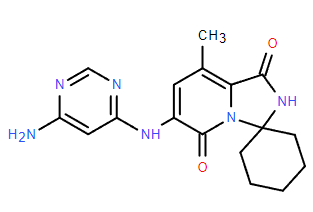
Code: EFT508
CAS:1849590-01-7
Company: eFFECTOR Therapeutics
Indications: colorectal cancer; diffuse large B-cell lymphoma; hepatocellular carcinoma; non-small cell lung cancer; prostate cancer; solid tumors; triple-negative breast cancer (Phase 2)
On April 4, 2024, eFFECTOR Therapeutics announced the results of the primary analysis of the randomized phase 2 KICKSTART trial, which tested tomivosertib or placebo in combination with pembrolizumab as first-line treatment in patients with non-small cell lung cancer (NSCLC) with PD-L1 ≥50%. Based on 36 events, the hazard ratio for progression-free survival (PFS, the primary endpoint of the study) was 0.62 (95% confidence interval 0.3 to 1.3) in favor of tomivosertib using a stratified Cox proportional risk model. the two-sided p-value for PFS based on the stratified log-rank test was 0.21, which did not meet the prespecified threshold of p≤0.2. Median PFS was 13.0 weeks in the tomivosertib combined pembrolizumab group and 11.7 weeks in the placebo combined pembrolizumab group. Overall survival outcomes remained immature, but no trend in favor of tomivosertib was observed. There were 67% grade 3 or higher treatment-emergent adverse events in the tomivosertib in combination with pembrolizumab group compared with 37% in the placebo in combination with pembrolizumab group.