Otsuka Pharmaceutical's third-generation BCR-ABL inhibitor declared for marketing in China and has been included in priority reviewName: ponatinib
CAS: 943319-70-8
On May 12, the CDE website showed that Otsuka Pharmaceutical's (Otsuka) ponatinib tablet (ponatinib) marketing application was accepted by the State Drug Administration.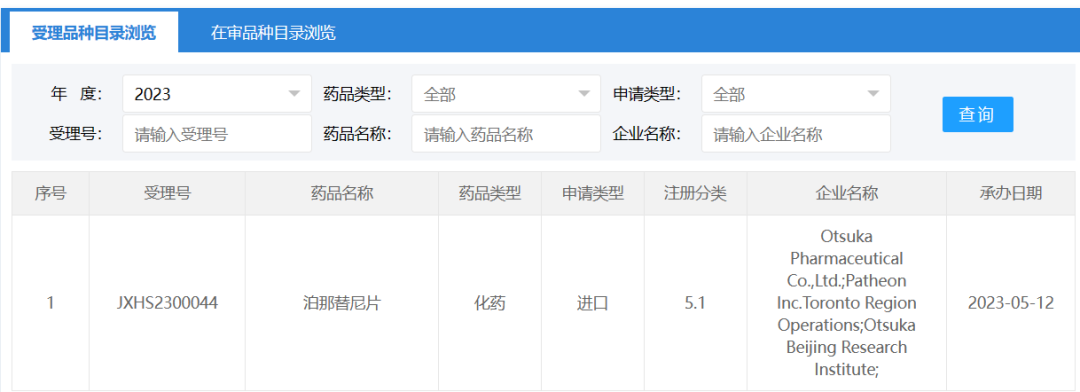 The drug marketing application has been submitted to CDE for priority review, and the proposed indications include chronic myeloid leukemia (CML) that has become resistant to or intolerable to prior treatments; relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL); and T315I-positive chronic myeloid leukemia or T315I-positive Philadelphia chromosome-positive acute lymphoblastic leukemia. Ponatinib tablets have previously been listed among the first batch of clinically urgent new medications available outside of China.
The drug marketing application has been submitted to CDE for priority review, and the proposed indications include chronic myeloid leukemia (CML) that has become resistant to or intolerable to prior treatments; relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL); and T315I-positive chronic myeloid leukemia or T315I-positive Philadelphia chromosome-positive acute lymphoblastic leukemia. Ponatinib tablets have previously been listed among the first batch of clinically urgent new medications available outside of China.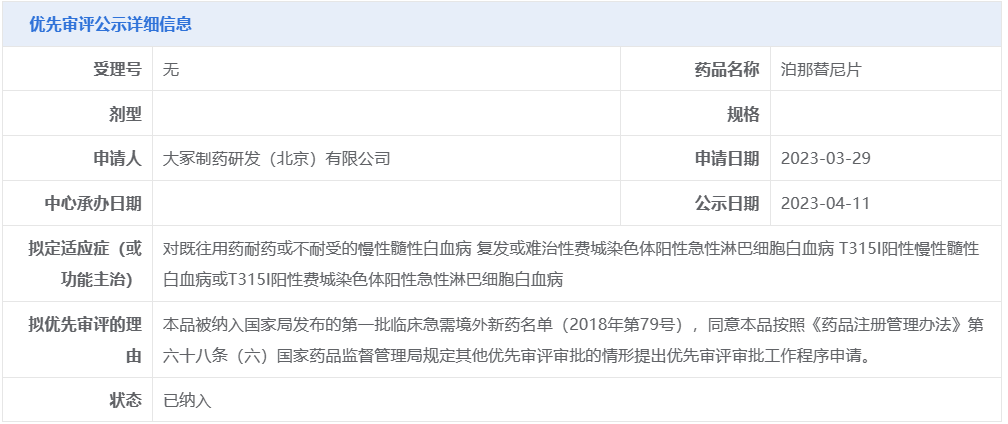 Ponatinib (trade name: Iclusig) is a third-generation inhibitor of BCR-ABL kinase, a tyrosine kinase aberrantly expressed in CML and Ph+ ALL. Ponatinib targets mutations in subtypes of BCR-ABL that are therapeutically resistant, including mutations in other authorized T315I mutations linked to resistance to other approved TKIs, in addition to the wild-type BCR-ABL that it carries.
Ponatinib (trade name: Iclusig) is a third-generation inhibitor of BCR-ABL kinase, a tyrosine kinase aberrantly expressed in CML and Ph+ ALL. Ponatinib targets mutations in subtypes of BCR-ABL that are therapeutically resistant, including mutations in other authorized T315I mutations linked to resistance to other approved TKIs, in addition to the wild-type BCR-ABL that it carries.
ARIAD Pharmaceuticals made the initial discovery of ponatinib, and on December 23, 2014, Otsuka Pharmaceutical and ARIAD Pharmaceuticals signed a licensing deal to commercialize ponatinib in Japan and nine other Asian nations as well as to finance ponatinib clinical trials in those nations. Takeda purchased ARIAD in January 2017, acquiring access to ponatinib and another brigatinib that inhibits ALK.
Ponatinib was first made available in the US in December 2012 for the treatment of adult patients with chronic-phase, accelerated-phase, or maternal cell-phase CML or Ph+ ALL who are resistant to or intolerable to prior tyrosine kinase inhibitors (TKI). In November 2016, the FDA approved its use for adult patients with T315I-positive CML or T315I-positive Ph+ ALL.
Nerik (orebatinib), a third-generation BCR-ABL inhibitor, was the first to receive Chinese regulatory approval and was added to the National Health Insurance Catalogue's 2022 version in November 2021.
Copyright © 2023 PHARMCUBE. All Rights Reserved.


