By sarms4muscle.com | 17 May 2023 | 0 Comments
The new drug narazaciclib CAS: 1357470-29-1
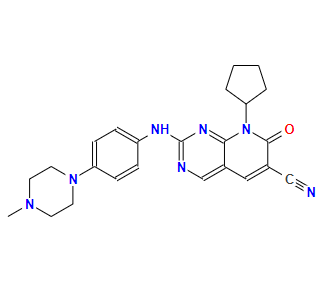
Name: Narazaciclib
CAS: 1357470-29-1
May 11, 2023 Onconova Therapeutics announced that the first patient has been dosed in the company's Phase 1/2a trial evaluating the efficacy of Narazaciclib in combination with letrozole for the treatment of recurrent metastatic low-grade endometrial like endometrial cancer (LGEEC).Preliminary Phase 1 clinical data are expected in Q4 2023 Preliminary Phase 1 clinical data are expected to be published in the fourth quarter of 2023.
The most typical cancer of the female reproductive system is endometrial cancer, which develops in the endometrium. The most prevalent subtype of endometrial cancer, accounting for almost 75% of all occurrences, is endometrial look-alike endometrial cancer. Letrozole may have anticancer effect in recurrent endometrial cancer when combined with CDK 4/6 inhibitors, according to prior research from randomized and single-arm trials1-3. Health authorities have not yet licensed any CDK 4/6 inhibitors for the treatment of endometrial malignancies. at the New York University Grossman School of Medicine, Bhavana Pothuri, PhD, is a professor of obstetrics and gynecology and the director of gynecologic oncology research; "The kinase inhibition profile of Narazaciclib suggests that due to reduced inhibitory activity against kinases associated with myelotoxicity and diarrhea, as well as against kinases associated with pre-tumor immunosuppression and cancer cell survival-related kinases, Narazachiclib can overcome each of these limitations," said David Perlmutter, director of the Perlmutter Cancer Center and principal investigator of the trial. This idea has been supported by data from in vitro and mouse cancer models, and I am eager to continue evaluating it in ongoing Phase 1/2a trials. synthesized medications sold to the general public.
About Phase 1/2a Clinical
In the Phase 1/2a trial, letrozole and narazacilib were used as a second- or third-line treatment for patients with recurrent metastatic LGEEC. This was an open-label, multicenter study. Letrozole and narazacilib were both given orally, continuously throughout the day. synthesized medications sold to the general public. The Phase 2 expansion cohort, which was intended to enroll about 30 patients, followed the Phase 1 dose-escalation phase, which was the first phase of the trial. To identify the appropriate Phase 2 dose of the combination (RP2D), the major goal of the Phase 1 of the experiment is to evaluate safety, tolerability, pharmacokinetics, and pharmacodynamics. The main goal of the Phase 2 phase is to evaluate the combination's effectiveness at RP2D using the 24-week progression-free survival metric. The estrogen/progesterone receptor status of the individuals will be noted as part of the exploratory objective. The trial will be carried out at locations including the lead investigator's site, NYU Langone Health, affiliated facilities of the MD Anderson Cancer Center, and the American Institute of Oncology.
CAS: 1357470-29-1
Molecular Formula: C24H27N7O
Formula Weight: 429.52

May 11, 2023 Onconova Therapeutics announced that the first patient has been dosed in the company's Phase 1/2a trial evaluating the efficacy of Narazaciclib in combination with letrozole for the treatment of recurrent metastatic low-grade endometrial like endometrial cancer (LGEEC).Preliminary Phase 1 clinical data are expected in Q4 2023 Preliminary Phase 1 clinical data are expected to be published in the fourth quarter of 2023.
The most typical cancer of the female reproductive system is endometrial cancer, which develops in the endometrium. The most prevalent subtype of endometrial cancer, accounting for almost 75% of all occurrences, is endometrial look-alike endometrial cancer. Letrozole may have anticancer effect in recurrent endometrial cancer when combined with CDK 4/6 inhibitors, according to prior research from randomized and single-arm trials1-3. Health authorities have not yet licensed any CDK 4/6 inhibitors for the treatment of endometrial malignancies. at the New York University Grossman School of Medicine, Bhavana Pothuri, PhD, is a professor of obstetrics and gynecology and the director of gynecologic oncology research; "The kinase inhibition profile of Narazaciclib suggests that due to reduced inhibitory activity against kinases associated with myelotoxicity and diarrhea, as well as against kinases associated with pre-tumor immunosuppression and cancer cell survival-related kinases, Narazachiclib can overcome each of these limitations," said David Perlmutter, director of the Perlmutter Cancer Center and principal investigator of the trial. This idea has been supported by data from in vitro and mouse cancer models, and I am eager to continue evaluating it in ongoing Phase 1/2a trials. synthesized medications sold to the general public.
About Phase 1/2a Clinical
In the Phase 1/2a trial, letrozole and narazacilib were used as a second- or third-line treatment for patients with recurrent metastatic LGEEC. This was an open-label, multicenter study. Letrozole and narazacilib were both given orally, continuously throughout the day. synthesized medications sold to the general public. The Phase 2 expansion cohort, which was intended to enroll about 30 patients, followed the Phase 1 dose-escalation phase, which was the first phase of the trial. To identify the appropriate Phase 2 dose of the combination (RP2D), the major goal of the Phase 1 of the experiment is to evaluate safety, tolerability, pharmacokinetics, and pharmacodynamics. The main goal of the Phase 2 phase is to evaluate the combination's effectiveness at RP2D using the 24-week progression-free survival metric. The estrogen/progesterone receptor status of the individuals will be noted as part of the exploratory objective. The trial will be carried out at locations including the lead investigator's site, NYU Langone Health, affiliated facilities of the MD Anderson Cancer Center, and the American Institute of Oncology.
Leave a Reply
Your email address will not be published.Required fields are marked. *
CATEGORIES